Each seizure and injunction conditions usually cause courtroom orders that need organizations to take numerous steps to proper CGMP violations, which may involve fixing facilities and products, increasing sanitation and cleanliness, performing supplemental screening to confirm excellent, and bettering worker instruction. FDA could also deliver prison circumstances thanks to CGMP violations, looking for fines and jail time.
Go undetected because of the limitations of current compendial bioburden tests in detecting this microbial genus
A separate drafting site is accessible with paragraph construction matching the Formal CFR formatting. If you work to get a Federal company, use this drafting web-site when drafting amendatory language for Federal regulations: change to eCFR drafting website.
(b) A consultant sample of models shall be gathered at the completion of finishing operations and shall be visually examined for accurate labeling.
(h) Printing devices on, or connected to, manufacturing lines accustomed to imprint labeling on the drug merchandise unit label or circumstance shall be monitored to assure that every one imprinting conforms towards the print laid out in the batch production report.
EMA is to blame for coordinating inspections of vaccine antigen manufacturing internet sites under the VAMF certification procedure.
(i) Three months once the expiration day with the drug product or service When the expiration dating period of the drug product or service is thirty days or significantly less; or
For those who have here concerns with the Company that issued the current doc make sure you Call the agency straight.
suggests the item specs and acceptance/rejection requirements, for example suitable quality level and unacceptable high-quality level, with the related sampling system, which have more info been necessary for earning a decision to just accept or reject a great deal or batch (or another easy subgroups of manufactured models).
Deciding on an product from full textual content search results will bring you to definitely These success. Pressing enter in the lookup box may also provide you to definitely search results. History and even more aspects are available in the Research & Navigation guide.
(6) Containers from which samples are actually taken shall be marked to indicate that samples have already been removed from them.
Compared into the GMP merchandise, cGMP items endure remarkably a lot more tests to prove the precision of it, as compared to that of GMP. It must undergo newer, and more in-depth screening and requirements than that of FDA GMP so the certification is exact and tested to get efficient.
Elements, drug merchandise containers, and closures authorised to be used shall be rotated so which the oldest authorised stock is utilized very first. Deviation from this necessity is permitted if such deviation is non permanent and correct.
Importers are accountable to make certain that the third country company These are importing from adjust to GMP.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now!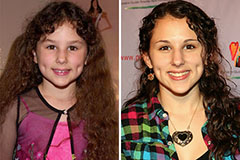 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Barry Watson Then & Now!
Barry Watson Then & Now! Raquel Welch Then & Now!
Raquel Welch Then & Now! Barbara Eden Then & Now!
Barbara Eden Then & Now!